길리어드 렘데시비르 관련주 임상결과 (파미셀, 신풍제약 등)
안녕하세요 건강한 투자자 부반 입니다.
부에~반하다

코로나19 바이러스가 한국에서 첫 확진환자가 발생한 날은 2020년 2월17일 오후 11시입니다.
그로부터 현재 2020년 4월18일 두달이 지나고 있는데요

전 세계적으로는 확진자가 218만명이 넘었고, 사망자또한 15만명이 넘었습니다.

국내 확진자 또한 1만명이 넘었고, 사망자는 230명이 넘었습니다
국내에서는 코로나19 바이러스가 점점 잡혀가는 모양세이지만 세계적으로는 미국, 일본 등에서는
매일매일 확진자와 사망자가 줄지 않고 있거나 더 늘고 있기도 합니다.
코로나19 바이러스가 잡히려면 사회적 거리두기, 국민들의 생활방역 등으로는 한계가 있기 때문에
하루빨리 코라나 바이러스 치료제가 나와야 하는 상황인데요
최근 질병관리본부에서도 하루빨리 코로나 바이러스 치료제를 개발하기 위해 박차를 가하고 있습니다.

그러던 중 2020년 4월16일 미국과 중국 그리고 한국 등에서 동시에 임상시험이 진행 중인
미국의 길리어드 사이언스에서 개발한 렘데시비르 (Remdesivir) 에 대한 긍정적인
임상 중간결과가 잇따라 공개되었습니다.
https://www.gilead.com/길리어드 사이언스 홈페이지
Gilead Sciences, Inc.
Together, we’re creating what’s possible. From science to community outreach, we’re committed to making a healthier world.
www.gilead.com

길리어드 사이언스 홈페이지에 방문하면 코로나19 COVID-19 유행성 관련 Daniel O'Day 다니엘 회장과
CEO의 조사 치료를 위한 임상 시험에 대한 업데이트 편지내용이 있습니다.

렘데시비르 치료제에 관한 길리어드 사이언스의 공식 입장 .
Gilead Sciences,
An Open Letter from our Chairman & CEO
Daniel O’Day - April 10, 2020
Earlier today, the New England Journal of Medicine (NEJM) published an analysis of the effects of our investigational medicine remdesivir on a small group of patients with severe symptoms of COVID-19.
These are patients who received treatment through the compassionate use program for remdesivir, which is for critically ill patients who are unable to take part in a clinical trial. The results, which cover 53 of the first patients to have been treated in the program, show that the majority demonstrated clinical improvement after taking remdesivir. We recognize the limitations of these compassionate use data from a purely investigational perspective, while knowing they are of the greatest significance for the patients whose symptoms improved. These early data from 53 patients have not been generated in a clinical trial and cover only a small portion of the critically ill patients who have been treated with remdesivir.
Remdesivir is an investigational treatment and has not been approved for use anywhere in the world. In the broader efforts to determine whether it is a safe and effective treatment, we have some way to go. Multiple clinical trials are underway across the world to build a complete picture of how remdesivir works in various contexts. These studies cover a range of patient populations across different demographics and with varying types of symptoms: moderate, severe where patients need oxygen support, and critical where medical ventilation is required. These patients all receive remdesivir through intravenous infusions in a hospital setting.
In studying remdesivir, the question is not just whether it is safe and effective against COVID-19 but in which patients it shows activity, how long should they receive treatment and at what stage of their disease would treatment be most beneficial. Many answers are needed, which is why we need multiple types of studies involving many types of patients.
Some of these answers will start to emerge in the coming weeks as we receive the first data from the various clinical trials underway.
Clinical trials for remdesivir
Seven clinical trials have been initiated to determine whether remdesivir is a safe and effective treatment for COVID-19. Each of these was set up with unprecedented speed thanks to the remarkable efforts of the various groups involved, as well as the level of knowledge we had on remdesivir.
To some extent, the trials have had to be adaptive in design as our understanding of the disease itself continues to evolve. The virus emerged and spread at an intense speed and everyone is working quickly to understand it. Our interpretation of the results will also be shaped by what we continue to learn about the disease.
The order in which the trials were initiated mirrors the path of the pandemic. China initiated the first two studies in early February for patients with severe and moderate symptoms of the disease. Since then, an additional five trials have been initiated around the world.
Two Phase 3 studies are being run by Gilead in areas with a high prevalence of COVID-19 in the United States, Asia and Europe. One of these is for patients with severe disease and the other studies remdesivir in patients with more moderate symptoms. One of the many questions that these studies aim to answer is whether treatment duration can be shortened from 10 days to 5 days. The severe arm fully enrolled the number of patients it was originally designed for and we have now expanded the study so that thousands more patients can participate, including those on mechanical ventilation.
The U.S National Institute of Allergy and Infectious Disease (NIAID) began a global trial on February 21. This trial randomly assigns patients to treatment with either remdesivir or with a placebo to enable a controlled comparison of outcomes. The trial is enrolling approximately 800 patients with a broad spectrum of symptoms.
The World Health Organization is also conducting a global trial, Solidarity, and the Inserm DisCoVeRy trial recently began in Europe. A summary of remdesivir trials with upcoming data readouts can be found here.
We know that there is tremendous interest around when the data from these trials will be available and what they will tell us about remdesivir. We feel the urgency as we wait for the science to speak. With every day that goes by, the desperate need to equip healthcare workers and their patients with a safe, effective treatment becomes more pressing. We are working with intense speed to determine whether remdesivir could be an option and we are committed to sharing information when it becomes available to us.
We expect that we will have preliminary data from the study of remdesivir in severe patients at the end of April and will work quickly to interpret and share the findings. The publication of data from the China remdesivir trials rests with the Chinese investigators, but we have been informed that the study in patients with severe symptoms was stopped due to stalled enrollment. We look forward to reviewing the published data when available. In May, we anticipate the initial data from the placebo-controlled NIAID trial as well as data from the Gilead study of patients with moderate symptoms of COVID-19.
To a large extent, the timelines are determined by epidemiology and the numerous challenges that come with studying a treatment for a newly emerged disease. As with so much in this pandemic, this is unchartered territory for many of us involved in the process.
Ongoing collaboration
While it may feel like a long wait for data given the urgency of the situation, it has been only two months since the first clinical trials began. Given that it can take a year or more to have the first clinical data for an investigational treatment, it is remarkable that we expect to have the first remdesivir trial data so soon.
This speed is the result of strong collaboration and immense dedication among the many groups involved, from regulatory authorities to hospital administrators, clinicians and study investigators. As with all the work on remdesivir, everyone is driven by the same sense of urgency and a commitment to maintaining scientific rigor throughout.
All of us at Gilead are grateful to the many groups and organizations who are collaborating to find answers on remdesivir and above all, to the physicians and patients involved in the clinical trials. When we talk about trial results, we tend to think in terms of numbers, trends and statistics. We realize that behind each of these numbers is a patient who has agreed to take part in a trial and share the data from their personal experience. It is thanks to the thousands of patients like these and the physicians who are treating them, that we will be able to determine whether remdesivir can be used safely and effectively for many more patients in the future.
Note regarding forward-looking statements.
길리어드 사이언스 회장 및 CEO의 공개 서한 내용을 보면 코로나19 바이러스 확진자
중환자 53명 대상으로 임상시험 결과 임상적 개선을 보였다고 합니다.
그러나 임상 시험용이기 때문에 아직 승인되지않았고, 임상 시험이 더 필요하다고 합니다.
길리어드 사이언스 회사 차원에서는 조심스러운 내용의 서한인데요
실제 뉴스기사에서는 인공호흡기를 끼던 일부 환자는 투약 하루만에 자가호흡이 가능해졌다
라고 하였고,
대부분 환자가 빠르게 효과를 보면서 치료 6일만에 퇴원까지 했다는 기사내용도 있습니다.
환자의 68%는 상태가 호전되었고, 결과적으로 완치된 환자는 47%에 달한다고 합니다.
길리어드 사이언스의 렘데시비르 임상은 현재 임상3상 시험을 진행중이기 때문에
임상3상까지 갔다는것은 FDA 승인이 날 가능성이 굉장히 높다는 뜻이기도 하죠
임상3상까지 간 렘데시비르 거기에 추가호재인 코로나 치료제로 각광받고 있기 때문에
주식시장에서는 엄청난 대형 호재로 봐도 무방합니다.
이런 이유로 길리어드 회사의 주가는 장외거래에서 16% 이상 급등하기도 했는데요
과연 우리나라 기업중에 렘데시비르와 관련된 기업들은 어떤기업들이 있을까요?
렘데시비르 관련기업은?
1. 파미셀

파미셀은 렘데시비르 주 원료인 '뉴클레오시드' 를 생산하는 기업입니다.
파미셀은 글로벌 진단용 및 의약용 뉴클레오시드 시장의 80% 이상을 점유하고 있기 때문에
렘데시비르와 관련된 가장 연관성이 큰 기업이라고 보여집니다.
다만 3월말부터 이미 상한가를 기록하면서 80%이상 주가가 뛴 상태이기 때문에
현재는 숨고르기 중으로 보여집니다. 과연 한번 더 뛰어 줄지 선택은?????????????
여러분의 몫으로 남겨두겠습니다.
2. 한올바이오파마
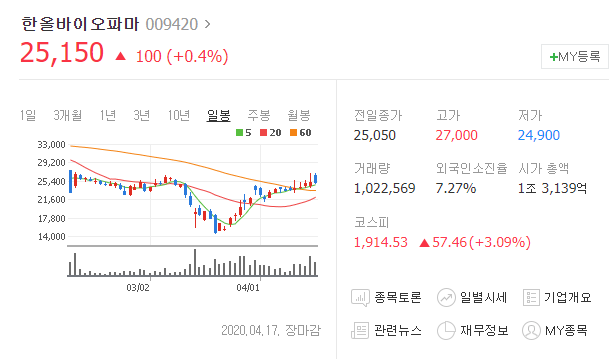
HIP Inc 라는 기업이 한올바이오파마의 자회사 인데요
면역항암 부분과 안구건조증과 같은 바이오 신약의 개발에 박차를 가하고 있는 회사 입니다.
특히, 메르스 치사율을 줄여주는 인터페론에 대한 특허 보유중인데요
렘데시비르가 메르스 치료제 라는건 다들 알고 계시죠?
1%의 조그만한 연관성만 보여도 주가에 영향을 주는게 바로 주식이죠
3. 에이프로젠제약

메르스 증상을 완화시켜주는 항바이러스에 대한 특허를 가진 기업이죠
의약품 제조, 판매를 주요 사업으로 영위하고 있고,
활발하게 운영이 되고 있습니다.
항생제와 소염진통제를 비롯한 다양한 전문의약품을 주력상품으로 생산 중인 기업이죠
에이프로젠제약도 메르스 증상을 완화? 시켜준다는 몇프로 안되는 연관성 있는 기업이네요 ㅋㅋ
그 외
신풍제약, 진원생명과학, 엑세스바이오 등의 기업이 렘데시비르, 메르스 치료제 등과 관련된
기업으로 테마로 묶여있는데요
코로나바이러스로 경제가 어려운 상황에서 주식투자도 리스크를 항상 최우선으로 둔
투자가 가장 안전합니다.
안전하게 리스크 관리하면서 성투하시길 바라며
투자의 판단은 언제나 여러분의 몫이라는거 아시죠?
내용이 유익하셨다면
구독 공감 알림 댓글
남겨주시면
저에게 아주큰 힘이 됩니다^_^